HILICpak VG-50 2D
主要分析應用:
Introduction Components of pharmaceutical products and food products often include high polar compounds. Those compounds are hardly retained under reversed phase mode, and to compromise the problem, methods using pre-column derivatization or addition of ion-pair reagent to the eluent are often applied. Drawbacks of them are the complicated and time taking process of the derivatization and increase of background level caused by the ion-pair reagent residues on the column and the flow-lines. Shodex™ HILICpak™ VG-50 series used in this application is a set of polymer-based amino columns which effectively separates various saccharides. The base of its packing material is polyvinyl alcohol, and a hydrophilic functional group is modified on the particle through a tertiary amine (Fig. 1).
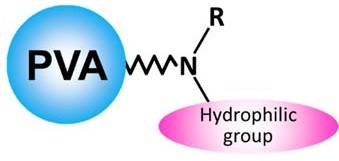 Fig.1 A schematic diagram of VG-50 packing material Fig.1 A schematic diagram of VG-50 packing materialWith some columns, reducing sugars form Schiff base with the packed material and are retained in the column. This does not occur with the HILICpak™ VG-50 series columns, and this leads the series to achieve high recovery rate. Moreover, column bleeding (elution of column packing material related debris) that is often observed with silica-based amino columns is rarely found with
the HILICpak™ VG-50 series columns, and consequently the related problems of increased background and/or ion suppression in MS are less likely to occur. Another advantage of the column over the silica-based amino column is that the HILICpak™ VG-50 series columns can be used under alkaline conditions (working pH range of 2-13). This lets a high sensitivity analysis of saccharides using negative mode in ESI-MS. Also, anionic compounds, such as organic saccharides, tends to be retained in the column when previously available methods are used, but use of alkaline conditions with the VG-50 will let them elute and makes it possible to analyze those organic saccharides.
This application introduces the results of not only saccharides, but including simultaneous analysis of saccharides, organic acids, and amino acids using a semi micro size column, Shodex™ HILICpak™ VG-50 2D, with LC/MS alkaline gradient conditions.
Experimental LC/MS system used was Shimadzu Nexera / LCMS-8030 Plus. The column used was Shodexä HILICpak™ VG-50 2D (2.0 mm I.D. x 150 mm; particle size 5 μm; pore size 100 Å). High pressure linear gradient with eluents of (A) either 0.1% or 0.5% ammonia water and (B) acetonitrile were used. The flow rate was set at either 0.2 or 0.3 mL/min. The column temperature was set at 30, 40, or 60 °C. ESI was used as a means of ionization and SIM or MRM mode was used for the detection. Specific analytical conditions used for each analysis will be mentioned with their results. It should be noted that the pH of 0.5% ammonia water is about 11.5. The LC/MS system used in the experiment was durable against the alkaline condition up to pH 13. Results and Discussion
1.LC/MS analysis of sugars 1-1. Neutral saccharides Fig. 2 shows the chromatograms for meso-erythritol, arabinose, xylose, fructose, mannose, glucose, sucrose, lactose, and maltose. Gradient elution of 0.1% ammonia water / acetonitrile was used. The neutral saccharides analyzed in this experiment can also be separated using water / acetonitrile as an eluent and results in similar resolution. However, addition of ammonia (i.e., analyzing under alkaline condition) promotes deprotonation during ESI which increases the sensitivity of negative ion detection. The peak height observed using ammonia water / acetonitrile eluent was three times higher than that of using water / acetonitrile eluent. The pH of 0.1% ammonium is about 11, which means that most silica-based LC columns cannot be used under this condition. This emphasizes an advantage of VG-50 2D, packed with polymer-based packing material, as it well-tolerates against the high pH eluent like the one used in this experiment. 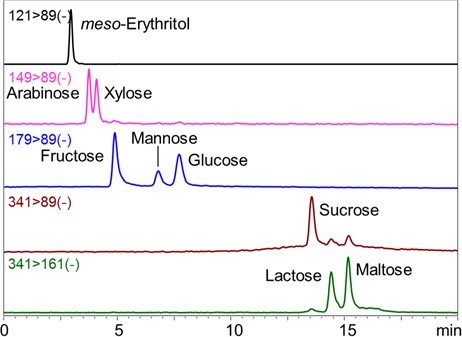 Fig. 2 Chromatograms of various neutral saccharides Sample: 50 ng/mL each in H2O/CH3CN=1/9, 5 μL Column: Shodex HILICpak VG-50 2D Eluent: (A) 0.1% NH3 aq. / (B) CH3CN Linear gradient; B%=95%(0-5 min) à 80%(15-20 min) Flow rate: 0.3 mL/min Detector: ESI-MS MRM (-) Column temp.: 60℃ 1-2. Acidic saccharides Use of water / acetonitrile eluent causes the acidic saccharides to be retained in the column by the influence of ionic adsorption. However, use of alkaline eluent will prevent the dissociation of amino function groups on the stationary phase, and thus saccharides will not be retained in the column. Fig. 3 shows the chromatograms of glucuronic acid and galacturonic acids. The degree of separation achieved here was better than that of previously available method using ion-exclusion chromatography. Using a high acetonitrile ratio is also advantageous for achieving a high sensitivity MS result. 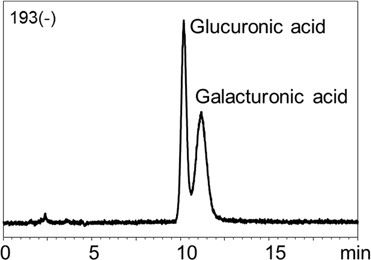 Fig.3 Chromatograms of two acidic saccharides Sample: 10 ng/mL each in H2O/CH3CN=1/4, 5 μL Column: Shodex HILICpak VG-50 2D Eluent: (A) 0.5% NH3 aq. / (B) CH3CN = 25 / 75 Flow rate: 0.2 mL/min Detector: ESI-MS SIM (-) Column temp.: 30℃
1-3. Glucose and gluconic acid 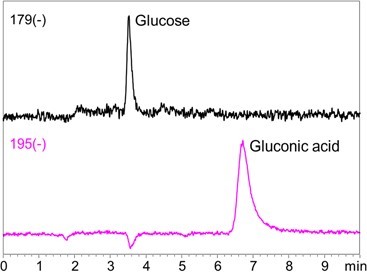 Fig.4 Chromatograms of glucose and gluconic acid Sample: 10 ng/mL each in H2O/CH3CN=1/4, 5 μL Column: Shodex HILICpak VG-50 2D Eluent: (A) 0.5% NH3 aq. / (B) CH3CN = 25 / 75 Flow rate: 0.2 mL/min Detector: ESI-MS SIM (-) Column temp.: 40℃ 1-4. Amino acids Fig. 5 shows chromatograms of N-acetylglucosamine and glucosamine. Since glucosamine contains an amino function group, higher sensitivity was achieved by monitoring protonated compound than monitoring its deprotonated compound. Amino sugars and their acetylated metabolites can also be analyzed under the alkaline condition (data not shown). 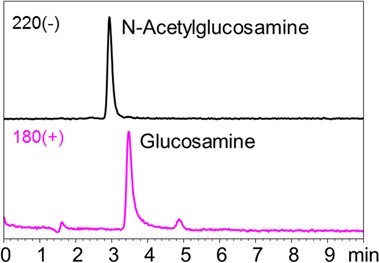 Fig. 5 Chromatograms of N-Acetylglucosamine and glucosamine Sample: 100 ng/mL each in H2O/CH3CN=1/4, 5 μL Column: Shodex HILICpak VG-50 2D Eluent: (A) 0.5% NH3 aq. / (B) CH3CN = 25 / 75 Flow rate: 0.2 mL/min Detector: ESI-MS SIM (+/-) Column temp.: 40℃ 2. Simultaneous LC/MS analysis of saccharides, organic acids, and amino acids
The result demonstrates its capability of analyzing some organic acids. The elution order of the organic acids was mono, di, and tribasic acids. It required to use approximately 0.5% ammonia to make the citric acid (a tribasic acid) to elute. Oxalic acid and citric acid are not retained well by ion- exclusion chromatography. However, the method developed here retains those acids well, and this helps them less likely to be affected by early eluting impurity peaks. It also demonstrated the feasibility of analyzing amino acids. The chromatograms showed hydrophobic amino acids to have tendencies of eluting earlier while acidic amino acids have tendencies to elute later. 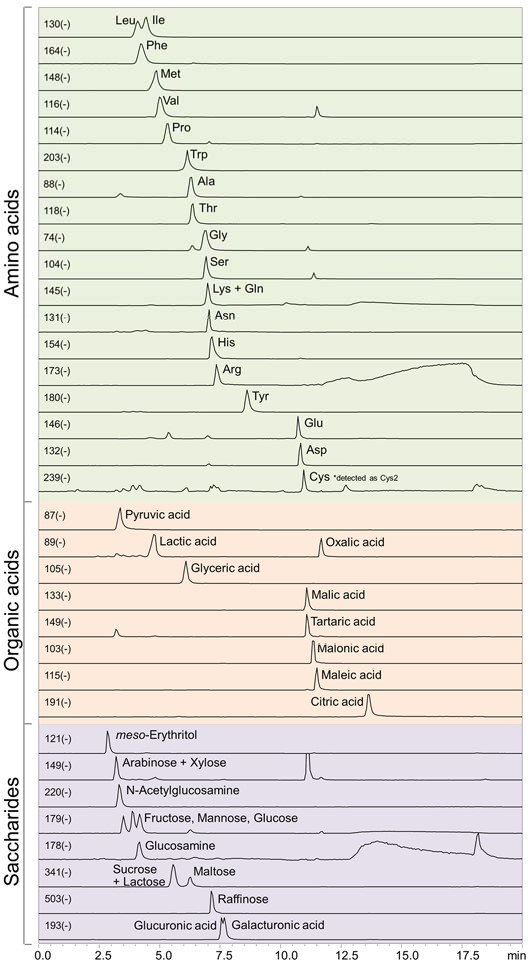 Fig. 6 Chromatograms showing the simultaneous LC/MS analysis of a mixture containing saccharides, organic acids, and amino acids Sample: 1 μg/mL each in H2O/CH3CN=1/4, 5 μL Column: Shodex HILICpak VG-50 2D
3.Application of the method for analyzing commercial energy drink Eluent: (A) 0.5% NH3 aq. / (B) CH3CN Linear gradient; B%=80%(0-2 min) à 10%(12-15 min) à 80%(15.01-20 min) Flow rate: 0.2 mL/min Detector: ESI-MS SIM (-) Column temp.: 40℃ Fig. 7 shows LC/MS analysis result of a commercially available energy drink. It demonstrated an effective simultaneous analysis of saccharides (fructose, glucose, sucrose), citric acid, and amino acids (isoleucine, phenylalanine, threonine, glutamic acid). Also, the method was feasible analyzing caffeine and water-soluble vitamins (nicotinamide, riboflavin, pyridoxine) present in the energy drink. 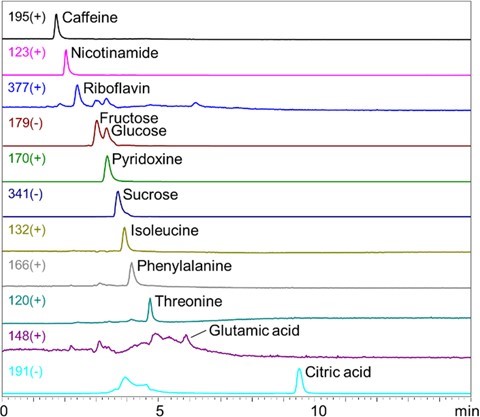  Fig.7 LC/MS analysis result of a commercial energy drink Eluent: (A) 0.5% NH3 aq. / (B) CH3CN High pressure linear gradient; B%=70%(0 min) à 10%(5-15 min) Flow rate: 0.2 mL/min Detector: ESI-MS SIM (+/-) Column temp.: 40℃ Conclusions A polymer-based amino column, Shodex™ HILICpak™ VG-50 2D, provides many advantageous analytical features when used under alkaline conditions. LC/MS with ammonia water / acetonitrile gradient elution is effective in providing good separation and high sensitivity analysis of various hydrophilic compounds. The method is feasible analyzing saccharides, organic acids, and amino acid simultaneously which was difficult by previously available methods. This can be achieved without using pre-column derivatization nor addition of ion- paring reagent. The alkaline condition promotes deprotonation of saccharides which makes it possible to monitor negative ions and contributes to the enhanced high sensitivity detection. The developed method showed its effectiveness in analyzing commercial energy drink. Not only monitoring saccharides, citric acid, and amino acids, the method demonstrated its ability to monitor caffeine and water soluble vitamins simultaneously. TA.NO.003.①18.D.JUL.P
|
立行科技 Analab Corporation
立行科技 Analab Corporation
| 電話 02-2776-6931 | 傳真 02-2776-6932 | sales@analab.com.tw | |||||
| 台北市中山區長安東路二段181號6樓之2 | |||||||



